1 M Copper Sulfate Solution

Older names for this compound include blue vitriol bluestone vitriol of copper and roman vitriol.
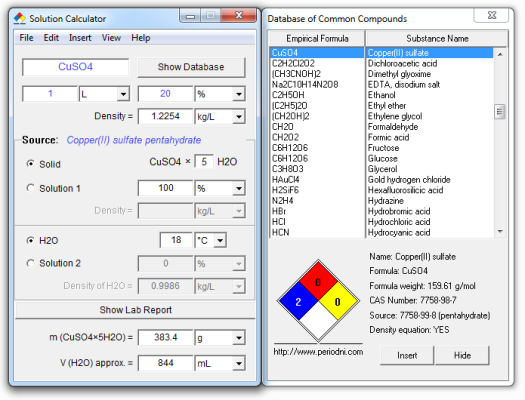
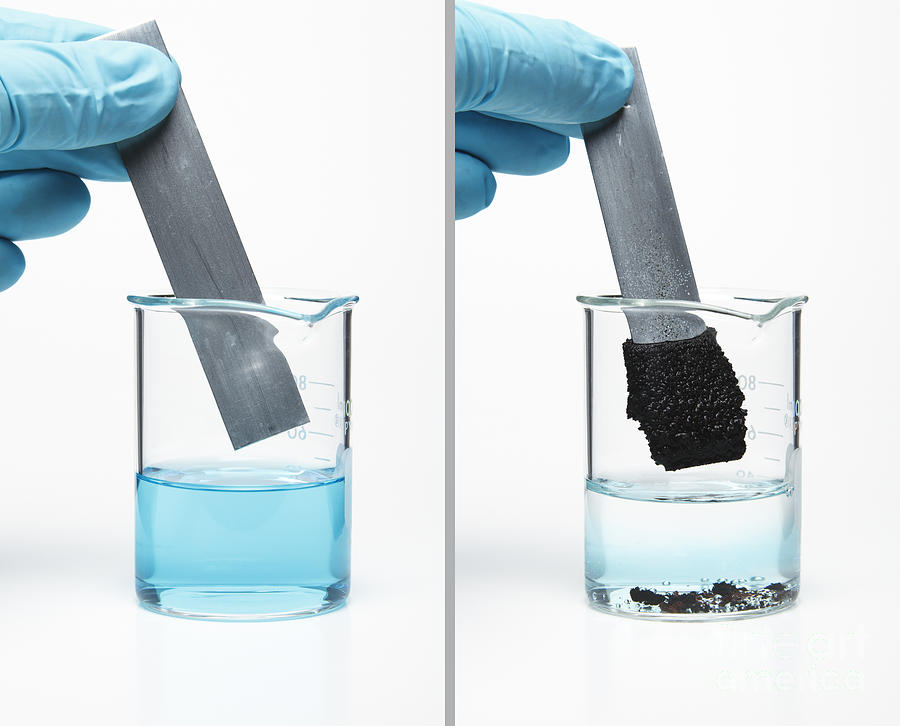
1 m copper sulfate solution. Bordeaux mixture is a suspension of cuso 4 and ca oh 2 used to prevent the growth of fungus on grapes melons and other berries. Cuso 4 copper ii sulfate is a non volatile odorless white crystalline substance. It usually forms crystals together with 5 molecules of water forming cuso 4 5h 2 o crystallohydrate. Record the mass of the copper sulfate pentahydrate along with the uncertainty and tolerance for the flask and balance.
1 weigh out enough copper sulfate pentahydrate cuso4 5h2o to create a 0 5 m copper sulfate solution with a final volume of 50 ml using a volumetric flask. When copper ii sulfate is obtained by crystallization from a water solution however five molecules of water are present for each molecule of copper ii sulfate. It is also used as an antiseptic. Appearance pale blue solution.
Solubility soluble in water and methanol. Molar mass cuso4 5h2o 249 68 g mol pentahydrate to make 100ml of 1 0m solutiuon weigh out 24 968g cuso5 5h2o and dissolve in water making final volume 100ml. It is toxic by ingestion and a strong irritant so care should be taken in handling. Vapor density air 1 n a.
Physical and chemical properties molecular formula cuso4 5h2o. It is widely used in agriculture. The pentahydrate cuso 4 5h 2 o the most commonly encountered salt is bright blue. The usual form of copper sulphate in the laboratory is the blue pentahydrate.
Specific gravity 1 19 g ml 20 c.